[2026 Cosmetic PIF Implementation Guide] Understand Regulations, Costs, and Penalties at a Glance | Professional OEM Assistance
As Taiwan’s cosmetic regulations increasingly align with international standards, the Product Information File (PIF) has become a crucial compliance requirement for all cosmetic brands and manufacturers. Starting from July 1, 2026, any general cosmetic product that fails to establish a PIF according to regulations may face fines of up to NT$1 million, along with risks of product suspension, recall, or business closure.
This article explains PIF from the perspective of OEM/ODM factories. It covers what a PIF is, key regulatory points for cosmetic PIFs, a checklist of PIF contents, cost structures, common penalties, and guidance for brands to determine the most suitable PIF setup method—reducing regulatory risk and accelerating product launch.
1. What is a PIF? A Complete Overview of Cosmetic Product Information Files
The PIF (Product Information File) is also known as the "Cosmetic Product Information File" and can be thought of as a cosmetic product’s “ID card plus full health report”. It systematically records the source of ingredients, manufacturing process, test data, and safety assessment results to demonstrate that the product is safe for consumers under normal or reasonably foreseeable use.
This system has been in place for many years in the European Union (EU) and ASEAN countries. Taiwan has officially adopted it as an important measure in transforming its cosmetic management system. The new system replaces the previous "pre-market approval for certain-use cosmetics" mechanism, shifting to brand self-management, combined with product registration and PIF creation, and subject to post-market inspections by authorities.
Why is creating a PIF necessary?
- Implements brand self-management and quality responsibility
- Enhances product safety and consumer trust
- Serves as a reference for regulatory inspections and market sampling
Who needs to establish a cosmetic PIF?
- Cosmetic manufacturers
- Cosmetic importers
- Cosmetic brands or responsible parties commissioning manufacturing
2. Key 2026 Cosmetic PIF Regulations: Penalties and Risks of Non-Compliance
According to Article 23 of the Cosmetic Hygiene and Safety Management Act, failure to establish or maintain a PIF according to regulations, or incomplete files, may result in the following risks:
| Action | Details |
|---|---|
| Fines | Fines ranging from NT$10,000 to NT$1,000,000, with potential repeated penalties per violation |
| Administrative Actions | Orders to stop sales/recall products, revoke registration, suspend or close business |
| Criminal Liability | Providing false information may result in criminal responsibility |
| Reputational Risk | Damage to brand reputation and loss of consumer trust |
| Market and Opportunity Loss | Unable to legally enter domestic or international markets, missing sales, export, exhibition, and channel opportunities |
3. Complete 16-Item Checklist for Cosmetic PIFs
To establish a compliant PIF, the following 16 core pieces of information must be included:
| No. | Required Item | Description |
|---|---|---|
| 1 | Basic Product Information | Product name, category, formulation type, intended use, manufacturer name and address, and information of the product manufacturer or importer |
| 2 | Proof of Product Registration | Valid documentation showing the product has been registered in the "Cosmetic Product Registration System," a first step before market launch |
| 3 | Full Ingredient List and Quantities | Complete listing of all ingredients and their exact percentages, consistent with the label's ingredient declaration |
| 4 | Labels, Leaflets, Packaging or Containers | Attach images of final product packaging, labels, and instruction leaflets to ensure compliance with regulations |
| 5 | GMP Compliance Documentation for Manufacturing Sites | Evidence or declaration confirming that the manufacturing facility meets Good Manufacturing Practice (GMP) standards |
| 6 | Manufacturing Method and Process | Summary of standard operating procedures (SOPs), including key steps like mixing, heating, and filling |
| 7 | Usage Instructions, Target Areas, Dosage, Frequency, and User Groups | Clearly define how to use the product, recommended amounts, and target groups (e.g., general skin types, avoid children) |
| 8 | Adverse Reaction Data | Record any consumer-reported reactions such as allergies or irritation, crucial for monitoring product safety |
| 9 | Physical and Chemical Properties of Product and Ingredients | Document physical and chemical characteristics (e.g., appearance, pH, purity) to ensure product quality standards |
| 10 | Toxicological Data of Ingredients | Toxicological assessment for each ingredient to ensure compliance with safety standards |
| 11 | Product Stability Test Report | Evidence that the product remains stable and maintains active ingredients throughout its shelf life |
| 12 | Microbiological Test Report | Third-party or lab testing confirming absence of harmful microorganisms (e.g., total microbial count, E. coli) |
| 13 | Preservative Efficacy Test Report | Proof that the product’s preservative system effectively inhibits microbial growth |
| 14 | Function Claim Evidence | If claiming specific functions (e.g., whitening, anti-wrinkle), provide experimental data, human tests, or literature supporting the claim |
| 15 | Packaging Material Information | Ensure containers or packaging materials do not chemically react with the product |
| 16 | Product Safety Summary | Comprehensive report drafted and signed by a qualified Safety Assessor (SA), officially confirming the product is safe for consumer use |
In practice, the most challenging items for brands are Item 9 (physical and chemical properties) and Items 10 & 16 (toxicology and SA safety assessment), as they are highly specialized and carry the highest risk of error.
Maywan offers a one-stop solution to simplify PIF creation, providing professional SA signing teams so brands can focus on market strategy while avoiding regulatory penalties.
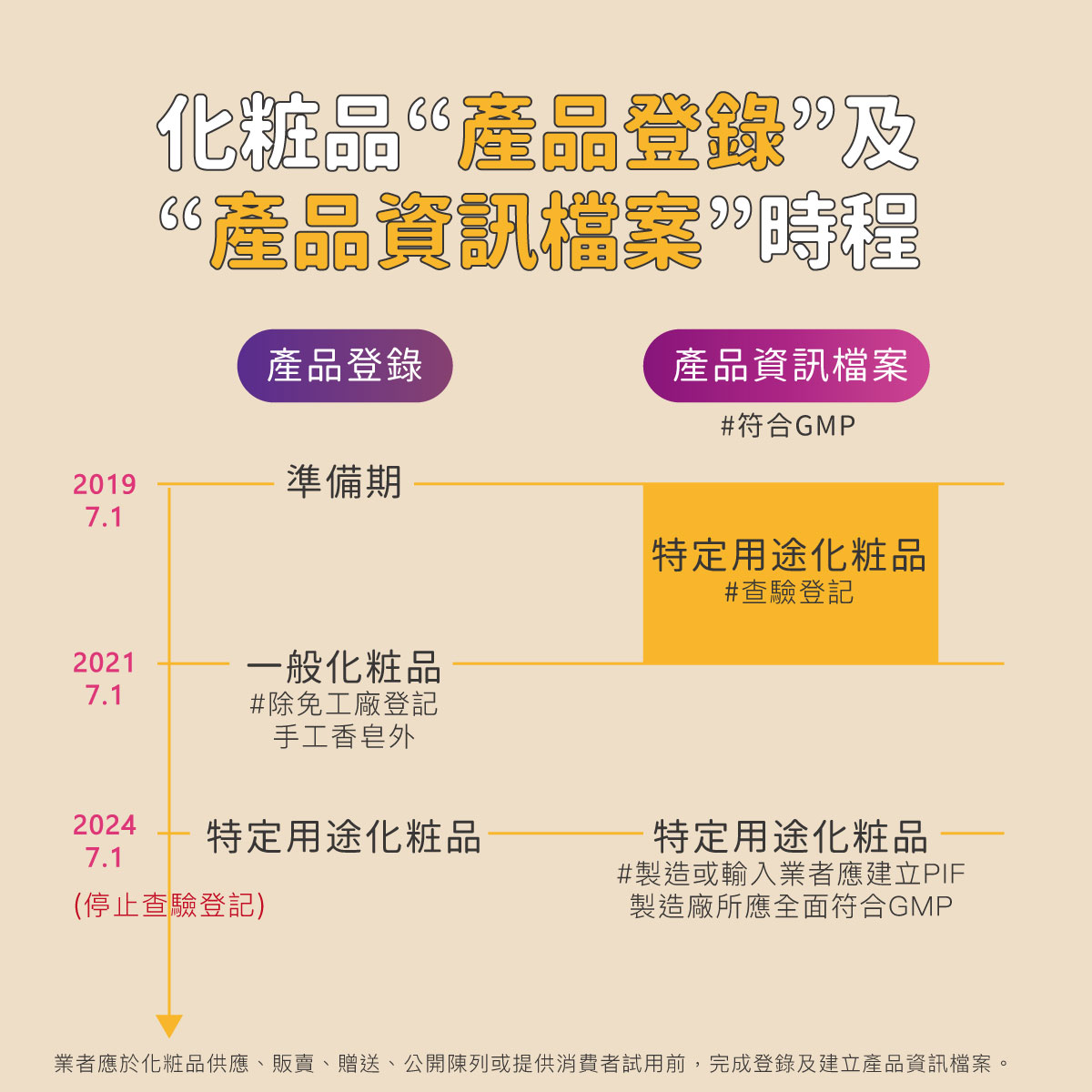
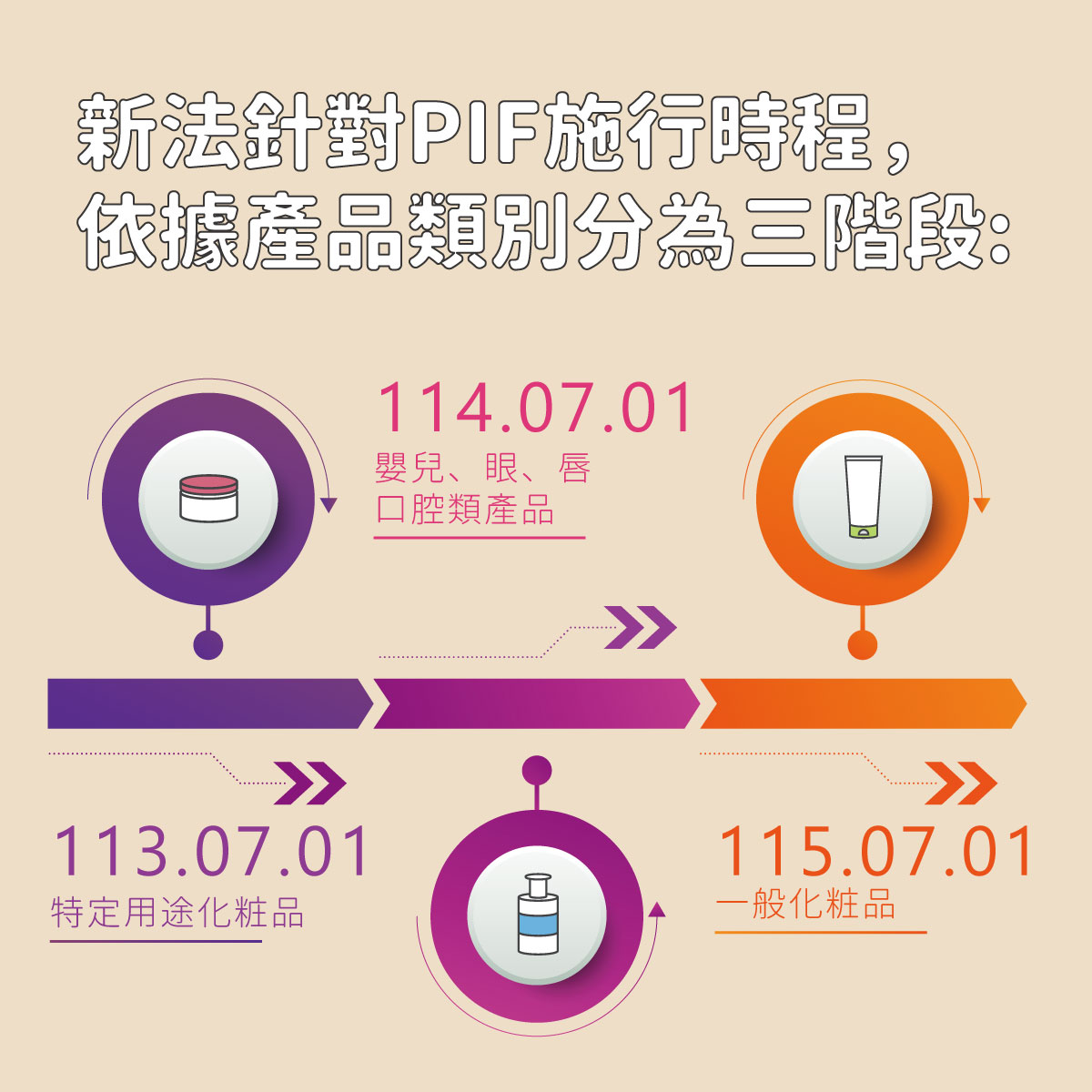
4. PIF Implementation Schedule and Product Categories
| Implementation Phase | Date | Applicable Scope |
|---|---|---|
| Phase 1 | 2024/07/01 | Certain-use cosmetics |
| Phase 2 | 2025/07/01 | Baby products, lip, eye products, non-medicated toothpaste and mouthwash |
| Phase 3 | 2026/07/01 | All general cosmetics (except solid handmade soap exempt from factory registration) |
The implementation of the PIF system represents Taiwan’s move toward an internationally aligned, comprehensive cosmetic safety management model.
5. How Much Does a PIF Cost? Setup Costs and Common Misconceptions
The cost of a cosmetic PIF typically includes the following items:
- Safety Assessor (SA) Signing Fee: Each product must be reviewed and signed by a qualified SA, making this one of the core costs.
Professional Reminder: Simply paying for an SA signature does not exempt the brand from responsibility. Legally, the primary responsibility remains with the brand. Choosing an experienced and system-compliant team is far more important than seeking the lowest fee. - Testing Fees: Includes necessary tests such as microbiological challenge tests, stability tests, and other lab analyses.
- Documentation Fees: If a PIF is outsourced to an agency, a service fee is usually charged for file organization and compilation.
Maywan, a professional OEM/ODM manufacturer of skincare in Tainan, Taiwan, provides a one-stop service for R&D, production, and PIF creation. In addition to a well-established R&D team, we also have professional Safety Assessors (SAs) to help brands organize PIF forms and compile complete PIFs. This reduces outsourcing costs and risks, ensuring the PIF is fully compliant.
6. Analysis of 3 Common PIF Setup Methods: Advantages and Disadvantages
| No. | Setup Method | Advantages | Disadvantages | Cost |
|---|---|---|---|---|
| 1 | Self-built PIF | Low cost, high control | Time-consuming, easy to miss details, requires qualified SA for safety report | Lowest cost, but requires internal time and labor investment |
| 2 | Outsourced PIF Agency | Professional and fast, regulatory consulting provided | High communication costs, may not fully understand product details, risk of errors | Highest cost |
| 3 | OEM-assisted PIF | Minimizes communication costs, familiar with formulation and processes, most complete data | Requires a capable and trustworthy OEM partner | Flexible cost depending on scope, most efficient |
PIF Video Reference: https://www.youtube.com/shorts/xJIRbH9v4Nk
7. Frequently Asked Questions (FAQ)
Q1: How long must a PIF be kept?
A: Files must be retained for at least 5 years for regulatory inspections.
Q2: Where can I find PIF file templates?
A: Authorities only provide a framework; actual content varies by product type. It’s recommended to consult partners with extensive PIF setup experience.
- Maywan provides one-stop services, assisting brands in organizing all 16 core PIF documents to lower the entry barrier for PIF setup
Q3: If the formula is slightly modified (e.g., changing fragrance or preservative source), does the PIF need to be updated?
A: Yes. PIF is a dynamic document. Any changes affecting ingredients or safety require an update. Even if the formula remains unchanged, it is recommended to review the safety assessment every 3–5 years to comply with the latest international banned substance standards.
Q4: Do samples, trial sizes, or gifts require a PIF?
A: Yes. Any product circulating in the market requires a PIF. Regulations apply whether the product is sold, given away, or used as a trial; safety requirements remain the same. Even a 5ml sample must be included in the PIF for that product.
Q5: What is the difference between PIF and cosmetic registration?
- Cosmetic Registration: Like “registering the household,” submitting basic product and ingredient information in the Cosmetic Product Registration System for regulatory oversight and transparency.
- PIF: Like a “background investigation,” including all scientific data, ingredient sources, and safety assessments. Both are mandatory; registration must be completed before preparing a PIF to legally sell the product.
In short, registration “notifies” the government about your product, while PIF “proves” your product is safe. Both must be completed to sell legally.
Q6: Does listing all ingredients and percentages in the PIF risk formula leakage?
A: No. The PIF is a non-public document. To prevent information leaks, “one-stop OEM manufacturing” is the most secure method:
- Data remains internal: Formulas circulate only within Maywan’s system, bypassing third-party agencies, eliminating external exposure points.
- Internal SA signing: Safety assessments are conducted by in-house professionals; core data does not need external review.
8. Conclusion: From Professional Haircare to Full-Range Skincare, Maywan Helps You Master PIF Compliance
With 2026 cosmetic regulations approaching, compliance equals competitiveness. Maywan, originating from professional salon haircare R&D and extending to high-performance skincare OEM, has over 26 years of OEM/ODM experience. As a leading Tainan cosmetic manufacturer, we provide ISO22716 and GMP-certified manufacturing environments, along with professional Safety Assessors. We offer a one-stop solution for brands, covering R&D, production, testing, and compiling all 16 core PIF documents. This minimizes the risk of regulatory penalties or fines and allows your products to quickly access international markets.
Contact Maywan today to have our professional team assist in achieving compliance, reducing risks, and focusing on brand development and market growth.
- Beauty and skincare trends, let's take a look at the mainstream ingredient analysis!
- Analysis of the Causes of Hair Loss and Hair Care Trends During Seasonal Changes: How Should Brands Develop Anti-hair Loss Products?
- Compliance with skincare OEM/ODM manufacturers can vary widely! Two essential principles for choosing a manufacturer
- Anti-hair Loss Product Formula Analysis: Three Popular Non-medicinal Hair Care Ingredients Recommended
- Say goodbye to a damp, hot, and oily scalp! Five key scalp care tips for a refreshing summer
- 2025 Hong Kong Beauty Expo (Cosmoprof Asia) | Post-Expo Highlights Summary
- Good Packaging Design Must Be Compliant: Understand the New Cosmetic Labeling Rules in 3 Minutes
- 【A Must-Save Guide for Startups】Skincare OEM Factory Collaboration Guide|Prepare These 5 Things for Smoother Product Development!


